Introduction: Historically, older patients (pts) with relapsed/refractory large B-cell lymphoma (R/R LBCL) were often deemed ineligible for curative-intent autologous stem cell transplant (ASCT) due to age and concern for increased toxicity related to comorbidities. We previously demonstrated in the pivotal ZUMA-7 study that older pts with R/R LBCL can safely receive axicabtagene ciloleucel (axi-cel) as second-line (2L) therapy with improved event-free survival (EFS), response rate, and quality of life compared with standard of care (SOC) based on results from the primary EFS analysis (Westin, et al. Clin Cancer Res. 2023). At a median follow-up of 47.2 months (mo), results from the ZUMA-7 primary overall survival (OS) analysis demonstrated superior OS in the intention-to-treat (ITT) analysis (hazard ratio [HR], 0.73; 95% CI, 0.54-0.98; stratified two-sided log-rank P=0.03; Westin, et al. N Engl J Med. 2023; NCT03391466). We now report updated efficacy and safety results from the primary OS analysis among ZUMA-7 pts aged ≥65 years (y) and ≥70 y.
Methods: Eligible pts were randomized 1:1 to axi-cel or SOC (2-3 cycles of platinum-based chemoimmunotherapy; responding pts proceeded to high-dose chemotherapy with ASCT). The primary OS analysis occurred 5 y after the first pt was randomized (01/25/2018) per protocol. Other endpoints included progression-free survival (PFS) per investigator assessment and safety. A planned subgroup analysis of pts aged ≥65 y was conducted in addition to an analysis for those ≥70 y. Multivariate analyses were performed to examine treatment efficacy with axi-cel compared with SOC after adjusting for multiple covariates, including sex, disease type, molecular subgroup, lactate dehydrogenase (LDH), tumor burden, and age; strata for these analyses included second-line age-adjusted International Prognostic Index (aaIPI), and relapsed vs refractory disease. Exploratory analyses were conducted to determine the association between OS and axi-cel product characteristics for pts aged ≥65 y.
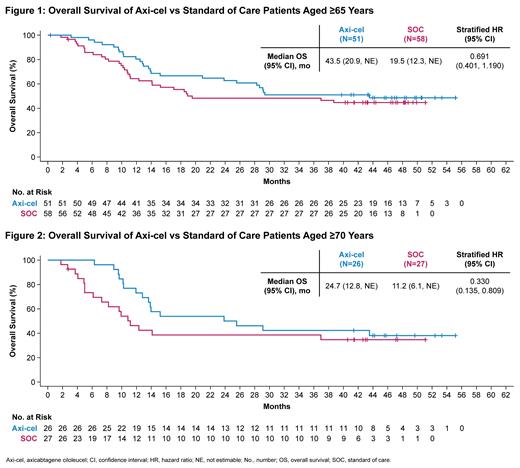
Results: A total of 109 pts were included in the analysis (axi-cel: 51 were ≥65 y, 26 of whom were ≥70 y, maximum age was 80 y; SOC: 58 were ≥65 y, 27 of whom were ≥70 y, maximum age was 81 y). Compared with SOC pts at baseline, more axi-cel pts had high-risk features, including aaIPI 2-3, elevated LDH, and high-grade B-cell lymphoma. At a median follow-up of 46.6 mo, OS was prolonged in the axicel vs SOC arm in pts aged ≥65 y (HR, 0.691; 95% CI, 0.401-1.190) and for those ≥70 y (HR, 0.330; 95% CI, 0.135-0.809). Similar results were observed using the piecewise Cox regression model. The median OS for axi-cel and SOC pts was 43.5 mo (95% CI, 20.9-not estimable [NE]) and 19.6 mo (95% CI, 12.3-NE), respectively, among those aged ≥65 y, and 24.7 mo (95% CI, 12.8-NE) and 11.2 mo (95% CI, 6.1-NE), respectively, among those aged ≥70 y. In the SOC arm, 57% and 52% of pts received subsequent cellular immunotherapy off protocol in pts aged ≥65 y and ≥70 y, respectively. Multivariate analyses demonstrated an even greater OS benefit with axi-cel over SOC when adjusting for differences in baseline characteristics in pts aged ≥65 y (HR, 0.526; 95% CI, 0.266-1.041) and in pts aged ≥70 y (HR, 0.184; 95% CI, 0.045-0.755). PFS assessed by investigator confirmed benefit of axi-cel over SOC in pts aged ≥65 y (HR, 0.406; 95% CI, 0.230-0.715) and in pts aged ≥70 y (HR, 0.206; 95% CI, 0.078-0.547). The median PFS for axi-cel and SOC pts was 28.7 mo (95% CI, 5.1-NE) and 5.0 mo (95% CI, 2.8-7.2), respectively, for those aged ≥65 y, and 11.4 mo (95% CI, 4.1-NE) and 2.7 mo (95% CI, 1.7-5.0), respectively, for those aged ≥70 y. No new treatment-related deaths occurred since the primary EFS analysis. There were no manufacturing failures for any pt who underwent leukapheresis. Similar associations between product characteristics and outcomes were observed among the elderly and overall populations, including improved OS associated with a greater (>median) proportion of juvenile or stem memory T-cell phenotype cells (CCR7+CD45RA+ T cells) in the axi-cel product among pts aged ≥65 y (HR, 0.369; 95% CI, 0.138-0.984).
Conclusion: Axi-cel as 2L therapy prolonged survival over SOC in pts aged ≥65 y, including in pts aged ≥70 y. These findings confirm that age alone should not be a barrier for consideration of CAR T-cell therapy, supporting the use of axi-cel as a curative-intent 2L therapeutic option for older pts with R/R LBCL.
Disclosures
Kersten:Novartis: Consultancy, Honoraria, Other: travel support; Miltenyi Biotech: Consultancy, Honoraria, Other: travel support; Adicet Bio: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Honoraria, Other: travel support, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Other: travel support, Research Funding; Takeda: Honoraria, Research Funding; BeiGene: Other: Travel support; Galapagos: Research Funding. Farooq:Kite, a Gilead Company: Honoraria; Caribou: Consultancy, Honoraria; MorphoSys: Consultancy; Regeneron: Research Funding. Locke:Cowen: Consultancy; BMS/Celgene: Consultancy, Other: Institutional ; EcoR1: Consultancy; Legend Biotech: Consultancy; CERo Therapeutics: Other: Institutional; Imedex: Other; A2: Consultancy, Other: Travel support; Allogene: Consultancy, Other: Institutional ; Cellular Biomedicine Group: Consultancy; Calibr: Consultancy; Wugen: Consultancy; Janssen: Consultancy; Novartis: Consultancy, Other: Institutional ; Gerson Lehrman Group (GLG): Consultancy; Aptitude Health: Other; bluebird bio: Consultancy, Other: Institutional ; Umoja: Consultancy; Caribou: Consultancy; Takeda: Consultancy; Daiichi Sankyo: Consultancy; Emerging Therapy Solutions: Consultancy; Iovance: Consultancy; Amgen: Consultancy; GammaDelta Therapeutics: Consultancy; ASH: Other; Clinical Care Options Oncology: Other; Sana: Consultancy; BioPharma Communications CARE Education: Other; Kite, a Gilead Company: Consultancy, Other: Institutional ; Individual Patents: Patents & Royalties: Several patents held by the institution in my name (unlicensed) in the field of cellular immunotherapy; National Cancer Institute: Other: Institutional ; Leukemia and Lymphoma Society: Other: Institutional ; Society for Immunotherapy of Cancer: Other: Institutional . Leslie:Celgene/ Bristol Myers Squibb: Other: Travel support, Speakers Bureau; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Speakers Bureau; LLS: Other: Educational role/ Leadership role in LLS light the night events (unpaid); BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Speakers Bureau; SeaGen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Speakers Bureau; Eli Lilly: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Speakers Bureau; Janssen/ J&J: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kite, a Gilead Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Speakers Bureau; Astrazeneca: Consultancy, Other: Travel support, Speakers Bureau; Beigene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Speakers Bureau; Janssen/PCYC: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Speakers Bureau; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Speakers Bureau; Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genmab: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CLL: Other: Educational role; LRF: Other: Educational role; Epizyme: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Speakers Bureau. Ghobadi:Genentech, Inc.: Research Funding; Wugen Inc: Consultancy; Kite/Gilead: Consultancy, Honoraria, Research Funding; CRISPR Therapeutics: Consultancy; BMS: Consultancy; Atara: Consultancy; Amgen: Consultancy, Research Funding. Miklos:A2 Biotherapeutics: Consultancy, Current holder of stock options in a privately-held company, Honoraria; Amgen: Consultancy, Honoraria; Adaptive Biotechnologies: Consultancy; Adicet: Research Funding; Bristol-Myers Squibb: Consultancy; NA: Patents & Royalties: cGVHD patent holder for Ibrutinib as cGVHD therapy but no compensation; Miltenyi: Consultancy, Research Funding; 2Seventy Bio: Research Funding; Allogene: Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Fate Therapeutics: Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Other: Travel support; Genentech: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Juno Therapeutics: Consultancy, Honoraria, Patents & Royalties: rights to royalties from Fred Hutch for patents licensed to Juno, Research Funding; Legend Biotech: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria; Mustang Bio: Consultancy, Honoraria; Navan Technologies: Consultancy, Current holder of stock options in a privately-held company, Honoraria; Novartis: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; Umoja: Consultancy, Honoraria; Bioline Rx: Membership on an entity's Board of Directors or advisory committees. Jacobson:Abbvie: Consultancy; ADC Therapeutics: Consultancy; AstraZeneca: Consultancy; Abintus Bio: Consultancy; Caribou Bio: Consultancy; Instil Bio: Consultancy; ImmPACT Bio: Consultancy; Daiichi-Sankyo: Consultancy; Ipsen: Consultancy; Morphosys: Consultancy; Synthekine: Consultancy; Pfizer: Research Funding; Miltenyi Biotec: Consultancy; Kite, a Gilead company: Consultancy, Research Funding; Novartis: Consultancy; Bristol Myers Squibb/Celgene: Consultancy. Munoz:Curio: Honoraria; OncView: Honoraria; MEI: Consultancy; Pfizer: Consultancy; Pharmacyclics/ Janssen: Consultancy, Research Funding, Speakers Bureau; Celgene: Research Funding; Morphosys/Incyte: Consultancy; Genentech/Roche: Consultancy, Research Funding, Speakers Bureau; Kite, a Gilead Company: Consultancy, Research Funding, Speakers Bureau; TG Therapeutics: Consultancy; Lilly/Loxo: Consultancy; Merck: Research Funding; Incyte: Research Funding; Portola: Research Funding; Physicians' Education Resource: Honoraria; Acrotech/Aurobindo: Consultancy, Speakers Bureau; Genmab: Consultancy; Seattle Genetics: Consultancy, Honoraria, Research Funding, Speakers Bureau; Karyopharm: Consultancy; Beigene: Consultancy, Research Funding, Speakers Bureau; Kyowa: Honoraria, Speakers Bureau; Epizyme: Consultancy; Alexion: Consultancy; ADC Therapeutics: Consultancy; AstraZeneca: Consultancy, Speakers Bureau; Millennium: Research Funding; Verastem: Consultancy, Speakers Bureau; Targeted Oncology: Honoraria; Celgene/ Bristol-Myers Squibb: Consultancy, Speakers Bureau; Bayer: Consultancy, Research Funding, Speakers Bureau; Pharmacyclics/Abbvie: Consultancy, Research Funding. Jaglowski:Takeda: Consultancy; Novartis: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; CRISPR: Consultancy; Caribou: Research Funding; Kite. a Gilead Company: Consultancy, Research Funding. Flinn:Seattle Genetics: Research Funding; BeiGene: Consultancy; Curis: Research Funding; CTI Biopharma: Research Funding; Epizyme: Research Funding; Fate Therapeutics: Research Funding; Forma Therapeutics: Research Funding; Forty Seven: Research Funding; IGM Biosciences: Research Funding; Incyte: Research Funding; Infinity Pharmaceuticals: Research Funding; Loxo: Research Funding; Marker Therapeutics: Research Funding; Millennium Pharmaceuticals: Research Funding; Nurix: Research Funding; Portola Pharmaceuticals: Research Funding; Rhizen Pharmaceuticals: Research Funding; Roche: Research Funding; Step Pharma: Research Funding; Tessa Therapeutics: Research Funding; Trillium Therapeutics: Research Funding; Constellation Pharmaceuticals: Research Funding; City of Hope National Medical Center: Research Funding; Celgene: Research Funding; CALGB: Research Funding; CALIBR: Research Funding; Bristol Myers Squibb: Research Funding; Biopath: Research Funding; ArQule: Research Funding; Agios: Research Funding; 2seventy bio: Research Funding; Xencor: Consultancy; Vincerx Pharma: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; Servier Pharmaceuticals: Consultancy; Secura Bio: Consultancy; Novartis: Consultancy, Research Funding; Myeloid Therapeutics: Consultancy, Research Funding; InnoCare Pharma: Consultancy, Research Funding; Genmab: Consultancy; AbbVie: Consultancy, Research Funding; Verastem: Consultancy, Research Funding; Pharmacyclics: Research Funding; Pfizer: Research Funding; Seagen: Research Funding; MorphoSys: Research Funding; Merck: Research Funding; Karyopharm: Research Funding; Juno: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Iksuda: Consultancy; Hutchison MediPharma: Consultancy; Great Point Partners: Consultancy; Gilead Sciences: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Century Therapeutics: Consultancy; BeiGene: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Acerta Pharma: Consultancy, Research Funding; Triphase Research & Development Corp.: Research Funding; Unum Therapeutics: Research Funding. van Meerten:Kite, a Gilead Company/ Gilead Sciences: Consultancy, Honoraria; Celgene/ BMS: Honoraria, Research Funding; Janssen: Consultancy; Gilead Sciences: Honoraria; Genentech: Research Funding. Perales:Miltenyi Biotec: Consultancy, Honoraria, Research Funding; Karyopharm: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; VectivBio AG: Consultancy, Honoraria; Merck: Consultancy, Honoraria; Allovir: Consultancy; BMS: Consultancy, Honoraria; Orcabio: Consultancy, Current equity holder in publicly-traded company, Honoraria; Exevir: Consultancy, Honoraria; Omeros: Consultancy, Current equity holder in publicly-traded company, Honoraria; MorphoSys: Consultancy, Honoraria; Kite: Consultancy, Honoraria, Research Funding; Medigene: Consultancy, Other; NexImmune: Consultancy, Current equity holder in publicly-traded company; Sellas Life Sciences: Consultancy; Syncopation: Honoraria; Vor Biopharma: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria, Research Funding; Celgene: Honoraria; Adicet: Honoraria; Servier: Other; Nektar Therapeutics: Consultancy, Honoraria, Research Funding; Miltenyi Biotec: Honoraria; Takeda: Consultancy, Honoraria; Cidara Therapeutics: Consultancy, Other; Caribou: Consultancy, Honoraria; Equillium: Consultancy, Honoraria; DSMB: Other; Allogene: Research Funding. Vandenberghe:Miltenyi Biotec: Consultancy; Janssen Biotech: Consultancy, Research Funding; Pfizer: Research Funding; Gilead Sciences: Consultancy, Other: Travel Support; Celgene/BMS: Consultancy; Kite, a Gilead Company: Consultancy, Other: Travel support; Novartis: Consultancy. Riedell:Pharmacyclics: Consultancy; Nkarta: Research Funding; Genmab: Consultancy; Karyopharm Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; MorphoSys: Research Funding; Genmab: Membership on an entity's Board of Directors or advisory committees; Nektar Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy; Celgene/ Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Xencor: Research Funding; Tessa Therapeutics: Research Funding; CVS Caremark: Consultancy; Calibr: Research Funding; Fate Therapeutics: Research Funding; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Intellia Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; CRISPR Therapeutics: Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Research Funding; Nurix Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sana Biotechnology: Consultancy; BeiGene: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees. Dorritie:BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab: Research Funding; Hoffman-LaRoche: Research Funding; Curio and Dava Oncology: Honoraria; Janssen: Research Funding; Kite, a Gilead Company: Research Funding; Genentech: Research Funding. Tzachanis:BMS: Consultancy, Research Funding. Vardhanabhuti:Kite, a Gilead Company: Current Employment, Current holder of stock options in a privately-held company, Research Funding. Du:Agios Pharmaceuticals: Consultancy; Kite, a Gilead Company: Consultancy. Filosto:Kite, a Gilead Company: Current Employment, Current holder of stock options in a privately-held company; Tusk Therapeutics: Other: Intellectual Property, Patents & Royalties. Shahani:Amgen: Speakers Bureau; Kite, a Gilead Company: Current Employment, Current holder of stock options in a privately-held company, Other. To:Kite, a Gilead Company: Current Employment; Gilead Sciences: Current holder of stock options in a privately-held company, Other. Westin:Genentech: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Research Funding; Nurix: Consultancy; Kymera: Research Funding; Morphosys/Incyte: Consultancy, Research Funding; Abbvie: Consultancy; AstraZeneca: Consultancy, Research Funding; MonteRosa: Consultancy; Calithera: Research Funding; SeaGen: Consultancy; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Kite/Gilead: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal